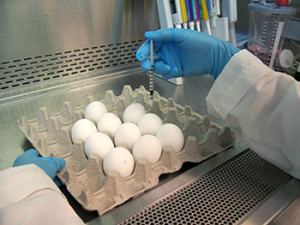
 While Flu vaccines are typically cultured using chicken eggs to grow the virus, a new technology was approved by the FDA last November. This is the first flu vaccine to use cell-based rather than egg-based technologies according to the FDA.
While Flu vaccines are typically cultured using chicken eggs to grow the virus, a new technology was approved by the FDA last November. This is the first flu vaccine to use cell-based rather than egg-based technologies according to the FDA.
Flucelvax, a new Flu vaccine approved by FDA in November 2012, is for use in people ages 18 and older. The unique part of the vaccine is that the manufacturing process for Flucelvax, while similar to the egg-based production, grows the virus strains included in the vaccine in cells of mammals.
Following on the approval of Flucelvax, another flu vaccine, known as Flublok, received FDA approval on January 16, 2013 and is manufactured using another new technology. For use in people 18 through 49 years of age, Flublok does not require the use of actual influenza viruses, and eggs are not used at any point in the manufacturing process.
According to the FDA, “Flublok uses an influenza virus protein that is made by genetically modifying a virus that infects insect cells to produce the flu vaccine protein. The protein, as in other flu vaccines, then triggers the immune system of the person receiving the vaccine to make protective antibodies.”
![Herbal Reference Substances are Key to Everyday Products <!-- AddThis Sharing Buttons above -->
<div class="addthis_toolbox addthis_default_style " addthis:url='http://newstaar.com/herbal-reference-substances-are-key-to-everyday-products/3512112/' >
<a class="addthis_button_facebook_like" fb:like:layout="button_count"></a>
<a class="addthis_button_tweet"></a>
<a class="addthis_button_pinterest_pinit"></a>
<a class="addthis_counter addthis_pill_style"></a>
</div>When it comes to quality control testing and the development of new products, Botanical Reference Materials (BRMs), also known as Herbal References are critically important. To help companies ultimately obtain all-important FDA approval, the Food and Drug Administration provides in its guidance a recommendation that […]<!-- AddThis Sharing Buttons below -->
<div class="addthis_toolbox addthis_default_style addthis_32x32_style" addthis:url='http://newstaar.com/herbal-reference-substances-are-key-to-everyday-products/3512112/' >
<a class="addthis_button_preferred_1"></a>
<a class="addthis_button_preferred_2"></a>
<a class="addthis_button_preferred_3"></a>
<a class="addthis_button_preferred_4"></a>
<a class="addthis_button_compact"></a>
<a class="addthis_counter addthis_bubble_style"></a>
</div>](http://newstaar.com/wp-content/uploads/2021/02/Achillea_millefolium_flowers-100x100.jpg)
![Quality Electrochemical Biosensors are Critical for Medical, Food and Chemical Industry <!-- AddThis Sharing Buttons above -->
<div class="addthis_toolbox addthis_default_style " addthis:url='http://newstaar.com/quality-electrochemical-biosensors-are-critical-for-medical-food-and-chemical-industry/3512086/' >
<a class="addthis_button_facebook_like" fb:like:layout="button_count"></a>
<a class="addthis_button_tweet"></a>
<a class="addthis_button_pinterest_pinit"></a>
<a class="addthis_counter addthis_pill_style"></a>
</div>A number of industries have, at their core, a need to frequent or even continuous analysis of biological media. These include the medical and pharmaceutical fields, biotech firms, and food and chemical companies. To maintain quality standards and develop new products, these industries rely heavily […]<!-- AddThis Sharing Buttons below -->
<div class="addthis_toolbox addthis_default_style addthis_32x32_style" addthis:url='http://newstaar.com/quality-electrochemical-biosensors-are-critical-for-medical-food-and-chemical-industry/3512086/' >
<a class="addthis_button_preferred_1"></a>
<a class="addthis_button_preferred_2"></a>
<a class="addthis_button_preferred_3"></a>
<a class="addthis_button_preferred_4"></a>
<a class="addthis_button_compact"></a>
<a class="addthis_counter addthis_bubble_style"></a>
</div>](http://newstaar.com/wp-content/uploads/2020/10/Electrochemical-Biosensor-100x100.jpg)
![Company Develops Industrial Mixers Well-Suited for both Fragile and Explosive Products <!-- AddThis Sharing Buttons above -->
<div class="addthis_toolbox addthis_default_style " addthis:url='http://newstaar.com/company-develops-industrial-mixers-well-suited-for-both-fragile-and-explosive-products/3512071/' >
<a class="addthis_button_facebook_like" fb:like:layout="button_count"></a>
<a class="addthis_button_tweet"></a>
<a class="addthis_button_pinterest_pinit"></a>
<a class="addthis_counter addthis_pill_style"></a>
</div>Industrial drum mixers are normally applied to blend mixes of varying viscosities such as adhesive slurries or cement. Some of these mixers have the capability of blending mixes of very different particle sizes such as fruit and ice cream, and gravel and cement slurry. The […]<!-- AddThis Sharing Buttons below -->
<div class="addthis_toolbox addthis_default_style addthis_32x32_style" addthis:url='http://newstaar.com/company-develops-industrial-mixers-well-suited-for-both-fragile-and-explosive-products/3512071/' >
<a class="addthis_button_preferred_1"></a>
<a class="addthis_button_preferred_2"></a>
<a class="addthis_button_preferred_3"></a>
<a class="addthis_button_preferred_4"></a>
<a class="addthis_button_compact"></a>
<a class="addthis_counter addthis_bubble_style"></a>
</div>](http://newstaar.com/wp-content/uploads/2020/06/bandeau-sofragir2-100x100.jpg)